Topics
USP7: A deubiquinating enzyme implicated in neurodevelopmental disorders
Proteins in our cells are constantly recycled and transported between various intracellular compartments using membrane-bounded carriers known as endosomes. Disrupting this flow of traffic upsets the delicate balance in our cells and can result in several diseases. One of the important cellular processes that ensures smooth flow of cellular traffic flows is the stability of the actin cytoskeleton. The Wiskott Aldrich syndrome protein and scar homologue (WASH) complex is one of the key regulators of the actin cytoskeleton.
Ubiquitination is one of the mechanisms by which cells control the stability and activity of proteins. E3 ubiquitin ligases add ubiquitin residues to substrate proteins, which can alter their fate and their activity in many ways. Depending on the molecular and cellular context, ubiquitination may label some proteins for degradation, whereas others may be recycled. Cellular homeostasis is achieved by deubiquitinating enzymes that reverses the action of ubiquitin ligases.
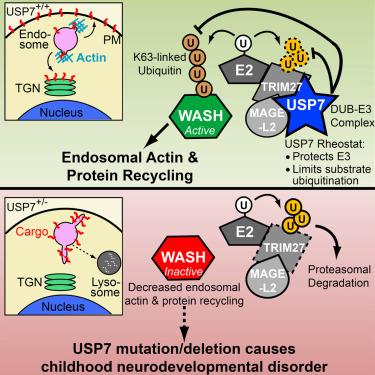
Addition of multiple ubiquitin moieties on WASH unlocks its default auto-inhibited state and makes it active. Previous studies from Dr. Ryan Potts’s lab at the University of Texas Southwestern Medical Center1 and others have shown that the MAGEL2-TRIM27 ubiquitin ligase complex is responsible for this poly-ubiquitination step. Interestingly, over the last few years, Dr. Christian Schaaf’s team at the Baylor College of Medicine and NRI have demonstrated that patients suffering from Prader-Willi (PWS) and Schaaf-Yang syndromes, neurodevelopmental disorders characterized by intellectual disability, autism and low muscle tone, have defective MAGEL2 function2.
In a recent study published in Molecular Cell3, these two groups in collaboration with others, have identified a novel role for Ubiquitin-specific peptidase 7 (USP7), a deubiquitinating enzyme, in PWS, Schaaf-Yang syndrome and a hitherto unidentified neurological disease.
The authors initiated this study to identify interacting partners of MAGEL2-TRIM27 and were surprised to isolate a deubiquinating enzyme, USP7 as one of the interactors. They found that USP7 exists in a stable complex with MAGEL2-TRIM27 and acts in concert to finely regulate WASH-mediated protein trafficking.
They found that USP7 performs two seemingly opposing yet extremely critical roles: it deubiquitinates WASH, but at the same time stabilizes TRIM27 by preventing its auto-degradation, which then, in turn, leads to ubiquitination of WASH, in favor of endosomal recycling pathways. This study provides novel insights that advances our understanding of a fundamental cell biological process.
The team was excited to note that this protein triad is indispensable in the neurons of hypothalamus, the region of the brain that is functionally defective in Prader-Willi and Schaaf-Yang syndromes. This indicated that defective function of this complex could perhaps be the underlying cause for some of the symptoms and encouraged them to look for outcomes of USP7 mutations.
The most exciting and astonishing aspect of this study is how quickly a basic science discovery made at the bench can be applied to patients, thanks mainly to rapid strides that we have made in sequencing technologies. After analyzing DNA sequences of tens of thousands of patients with unidentified neurodevelopmental disorders, they found seven individuals with mutations in USP7. Amazingly, all these individuals exhibited characteristic hallmarks of Schaaf-Yang syndrome with varying severity, clearly highlighting the close molecular and clinical relationship between MAGEL2 and USP7.
In summary, this study has identified MAGEL2-TRIM27-USP7 as the molecular nexus, and therefore, a potential therapeutic target for the various neurobehavioral symptoms experienced by patients suffering from Prader-Willi or Schaaf-Yang syndromes, and related neurodevelopmental disorders.
References:
1. Hao et al., Cell. 2013 Feb 28; 152(5): 1051–1064. Regulation of WASH-Dependent Actin Polymerization and Protein Trafficking by Ubiquitination
2. Schaaf et al., Nat Genet. 2013 Nov;45(11):1405-8. Truncating mutations of MAGEL2 cause Prader-Willi phenotypes and autism.
3. Yi-Heng Hao et al., Mol. Cell. 2015. DOI: http://dx.doi.org/10.1016/j.molcel.2015.07.033. USP7 Acts as a Molecular Rheostat to Promote WASH-Dependent Endosomal Protein Recycling and Is Mutated in a Human Neurodevelopmental Disorder




