Topics
A novel neuropeptide signaling integrates adult-born neurons into existing circuitries
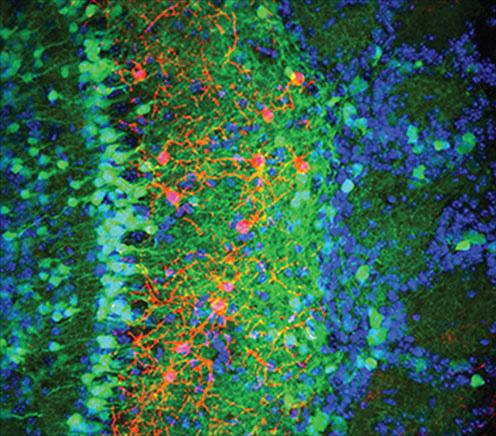
Until a few decades ago, it was largely believed that new neurons are only formed in the developing brain. It is now known that certain regions of the adult mammalian brain have the ability to generate new neurons throughout life. Yet, the cellular and molecular cues that direct the newly born neurons to their appropriate locations, facilitate their integration into an already existing complex network and promote their survival are not known.
Identifying mechanisms that promote synaptogenesis and proper circuit integration of adult-born neurons has been the subject of investigation in the laboratory of Dr. Benjamin Arenkiel, assistant professor of neuroscience at Baylor College of Medicine and the Jan and Dan Duncan Neurological Research Institute (NRI) at Texas Children’s Hospital.
This week, in Developmental Cell, Dr. Arenkiel and his team report a novel role for a small, soluble neuropeptide, Corticotrophin Release Hormone (CRH) in promoting synapse formation and circuit integration of neurons generated in the adult mouse brain. Traditionally, CRH has been studied as a hypothalamic stress hormone. However, it is now known that CRH is released by various neurons throughout the brain.
Specifically, the researchers found that CRH is secreted by a group of interneurons in the olfactory bulb and directly acts on newly generated neurons that express the cognate CRH receptor during a critical window for cell survival and synapse formation. This extrahypothalamic local CRH signaling facilitates integration of adult-born neurons by promoting their survival and acts as a value cue to guide these neurons into their proper place within the established network of neuronal connections.
Trans-synaptic viral tracing identifies functional circuitry in the olfactory bulb
Neurogenesis is the process by which new neurons are formed and integrated into neural circuits. During embryogenesis, neurons wire up easily to form new circuitries, which are highly adaptable. However, in the adult brain, newly generated neurons face unique challenges which are finding their right connectivity partners and seamlessly integrating into an already existing hardwired complex circuit. Understanding how this fundamental process occurs in the adult brain will undoubtedly provide valuable insights for the development of novel cell and circuit-based therapies for damaged or diseased nervous tissue.
The authors used the olfactory system of the adult mice as a model to study adult neurogenesis because it is one of the few regions of the adult brain where new neurons are continuously generated and integrated into existing networks.
“The impetus for embarking on this research was to identify the causative signals and steps that help to build and maintain synapses. We had a clue from rodents that neural activity somehow triggers the formation of synapses. The project started with the goal of trying to find the inputs that confer synaptogenic cues on adult-born neurons during their process of integration,” Dr. Arenkiel.
Dr. Arenkiel’s team employed an elegant tool that has been recently developed to trace functional connections between neurons. They selectively injected adult-born neurons in the olfactory bulb with a modified, non-infectious form of rabies virus. This recombinant form of rabies virus is capable of jumping between initially infected cells and their immediate presynaptic partner cells. Using this and other techniques, the researchers made the novel observation that adult-born neurons received extensive inputs from a specific group of local interneurons capable of releasing the neuropeptide CRH.
Local Corticotrophin release hormone (CRH) signaling promotes survival and connectivity of adult-born cells
Interestingly, Isabella Garcia, an MD/PhD student in Dr. Arenkiel‘s lab found that expression of CRH receptor expression was dynamically regulated to coincide with critical windows when synapse formation in these neurons was at their peak.
Since CRH and its receptor were present at the right time and at the place, the next important question that the researchers asked was: Is local CRH signaling required for the circuit integration of adult-born neurons?
The answer was a resounding yes.
In transgenic mice lacking copies of CRH or its receptor, many adult-born neurons died soon after birth and the ones that survived had fewer synapses. These data demonstrated that CRH signaling is critical for the survival and formation of synapses by adult-born neurons. Consistent with this, specific enrichment of an active form of the CRH receptor in the newly born neurons resulted in additional functional synapses. Taken together, it was clear that the activation of local CRH signaling had successfully integrated the newly generated neurons into the pre-established network within the adult brain.
This is the first time that local extrahypothalamic CRH signaling has been implicated in the integration of adult-born neurons. Usually, in fact, neuropeptides are primarily used as tools to mark different classes of interneurons throughout the brain and very little is known about their function.
Local CRH signaling-directed circuit plasticity: A valuable therapeutic tool
The team found that this unique ability of local CRH signaling to control synaptogenesis and circuit plasticity could also be externally regulated via neural activity. The technologies applied in this study have opened up future avenues of research on the potential of manipulating extrahypothalamic neuropeptide signaling, individually or in a combinatorial manner to repair adult neuronal circuitry. Neuropeptides act differently from classical neurotransmitters and play a neuromodulatory role. This study suggests that if researchers could find ways to regulate their activity in vivo, they could repair genetically defective or injured neurons and perhaps provide avenues for the survival and proper integration of neurons in stem cell therapies.
“A common theme that recurs among neurological diseases, neurodegenerative disease, trauma or neuropsychiatric diseases is that neurons die or are dysfunctional. The impetus for our research efforts is to find alternative approaches to repair the adult nervous tissue,” says Dr. Arenkiel.




