Topics
A kinase that restores memory and cognitive defects in a mouse model of Alzheimer's disease discovered
In an exciting study published in the journal Neuron, Drs. Huda Zoghbi and Juan Botas, principal investigators at the Neurological Research Institute at Texas Children’s Hospital and professors at the Baylor College of Medicine, have identified a kinase whose partial inhibition reduced aberrant tau aggregates and reversed memory deficits in a mouse model of neurodegeneration.
Accumulation and aggregation of usually soluble proteins such as tau and amyloid beta are the pathological hallmarks of many neurodegenerative diseases such as Alzheimer’s disease (AD). Ultimately, neurons with tau aggregates die in massive numbers.
It is widely believed that tau aggregates are associated with neuronal death although the exact causative mechanisms are not clear. Previous studies have shown that reducing tau levels can successfully restore memory deficits and cognitive impairments but the challenge was to lower tau using a strategy that also lends itself to the development of a safe and effective therapeutic agent.
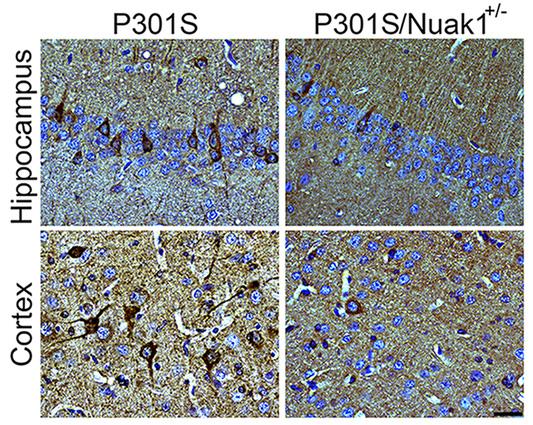
In this study, researchers employed an innovative approach to identify novel genetic modifiers of tauopathy. They performed a stringent parallel cross-species screen of approximately 600 known kinases in a human cell line and fruit flies that overexpressed tau.
Interestingly, they found that partial inhibition of a kinase called Nuak 1 reduced aberrant tau aggregates in both human cells and fruit flies. Most importantly, 50 percent reduction of Nuak 1 was sufficient to improve spatial memory and learning deficits in a mouse model of tauopathy. Also, it was encouraging to note that partial short-term inhibition of Nuak 1 did not result in any adverse side effects in mice overexpressing tau.
Thus, this study has identified a novel regulator of tau that alleviates memory and cognitive impairments in animal models. Hence, the researchers propose that Nuak 1 should be explored as a potential therapeutic target for tauopathic neurodegenerative diseases.
Another unique feature of this study is it has identified an evolutionarily conserved target that functions at the early stages of disease pathogenesis, a point at which tau aggregates begin to accumulate in overwhelming amounts, well beyond a neuron’s ability to clean up excessive protein accumulation.
Hence, it is tempting to speculate that partial inhibition of Nuak 1 in AD patients may also reverse cognitive and memory deficits, and if administered early on to high-risk patients, could perhaps even halt the development of certain neurodegenerative diseases such as AD.





